Atoms and molecules are the fundamental building blocks of matter.
Different kind of element is formed due to different atoms constituting
them. Atoms can be further divided into sub atomic particles. There are
three sub-atomic particles of an atom are:
(i) electrons, (ii) protons and (iii) neutrons.
Charged particles in matters
When we rub two objects together then they get electrically charged.
This is due subatomic particles that is the electron which are indivisible
particles of an atom was discovered by JJ Thomson in the year of 1990.
Further on research it was discovered that an atom consists of electron and
another subatomic particle that is the proton. the protons in an atom is
opposite in charge and equal in magnitude of that of the electrons.
Electron is negatively charged and proton is positively charged.
Mass of proton is taken as 1 unit charged that is +1, virus of an electron
mass is considered negligible and charge is -1. It is observed that the and
electrons mutually balance their charges with the protons. Protons are
present in the interior of the atom whereas the electrons are present in
the exterior part of an atom and can easily be peeled off from the atom.
Structure of an atom
The discovery of electron and proton inside the atom led to the failure of
Dalton's atomic theory and the belief of atom as indivisible and
indestructible was claimed false.
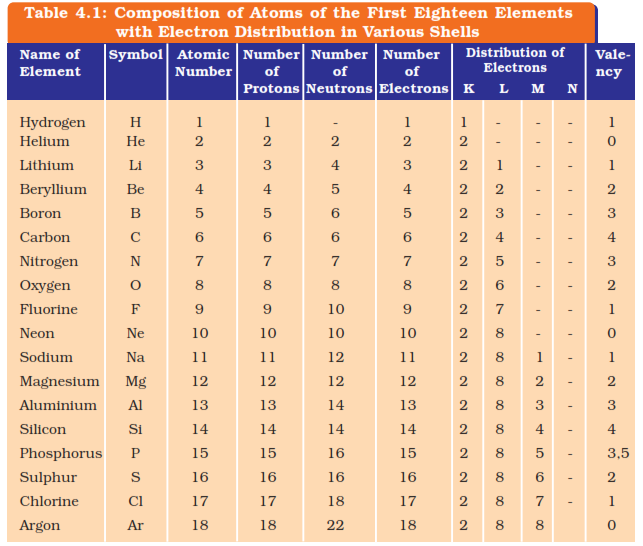
JJ. Thomson's model of an atom
In this model of an atom it was told as the electrons were spread in a
sphere of positive charge as can be seen in a watermelon the whole red part
will be supposed as the positive charge and the seeds are supposed to be
the electrons.
He proposed
An atom consists of a positively charged sphere and the electrons are
embedded in it.
Negative and positive charge are equal in magnitude and makes the atom
neutral as a whole.
Rutherford Model of an atom
In this model the scientist experimented by dropping Alpha particles on a
thin gold foil. Gold foil was selected because it can be very thin layer
which was about 1000 atoms thick. Alpha particles where doubly charged
Helium ions since they have an atomic mass of 4 while moving fast they will
have considerable amount of energy and it can be expected that the Alpha
particles will be deflected by the sub atomic particles in Gold atoms.
Observations of the experiment
Most fast-moving Alpha particle passed Straight Through the gold foil.
Some of Alpha particles get deflected by the foil by small angles.
Surprisingly, out of every 12000 particles appeared 1 particle that
rebounded at an angle of 180 degree.
From the experiment Rutherford concluded that
Most of the space inside is empty because Alpha particles passed through
the gold foil.
Very few particles deflected indicating positive charge occupies very
little space in an atom.
As a very small fraction of particles were reflected by an angle of 180
degree it indicates positive charge were concentrated in small volume
within the atoms.
Rutherford also put forward the Nuclear Model of an atom
There is a positively charged center in an atom called the nucleus and the
nucleus resides almost all the mass of an atom.
Electrons revolve around the nucleus in well-defined orbits.
Size of nucleus is very small compared to the size of atoms.
Drawback of Rutherford Model
orbital Revolution will not be stable. Because particles in circular orbit
would undergo acceleration and during this acceleration particles may
radiate energy thus lose energy and finally fall off into the nucleus of
the atom. Hence, matter would not exist in the form we know.
Bohr model of atom
In this model he described the following:
Only certain special orbits known as discrete orbit of electron are allowed
inside the atom.
While revolving in discrete Orbit the electrons do not radiate energy.
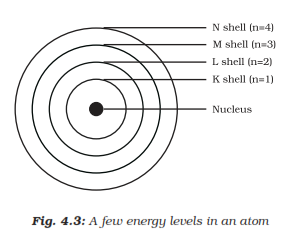
Orbits or shell are called the energy levels of an atom. the orbits or
shell are represented by the letters K,L,M,N... or by the numbers
n=1,2,3,4...
Neutron
Neutrons are sub atomic particle discovered by J. Chadwick which has no
charge and mass nearly equal to proton. Neutron is generally represented by
"n". Therefore, the mass of an atom is the sum of mass of protons and
neutrons present in the nucleus.
Electron distribution in different Orbit
Electron are distributed into different orbits as suggested by Bohr and
Bury.
Rules for writing the number different energy level are as follows:
The maximum number of electron present in a shell is given by the formula
2n2. Where "n" is the orbit number or energy level index.
The maximum number of electrons that can be present in the outermost orbit
is 8.
Electrons are not accommodated in a given shell unless the inner shell are
filled with electrons.
Valency
The total number of electrons present in the outermost shell of an atom are
known as the valence electrons and we also know that the total number of
electrons in the outermost shell cannot be more than 8. Valency gives the
combining capacity of an atom so if the outermost shell has 8 electrons the
combining capacity or valency is zero.
Combining capacity
The combining capacity of an atom creates the tendency of an atom to react
with another atom of same or different element to form a molecule. The
tendency of this reaction is to attend a full filled outermost shell so
that it can have 8 electrons in the outermost shell and achieve an octet
which is stable.
So, the number of electrons gained, lost or shared from the outermost shell
gives us directly the combining capacity of that element that is the
valency of the atom.
The valency of the atom of an element is written in two ways. when the
number of electrons is less in the outermost shell then the valency is
written as 1,2,3,4. And if the number of electrons is closed to 8 that is
5,6,7,8 then the valency determined in opposite direction that is, valency
is 1 for 7 electrons, 0 for 8 electrons in the outermost shell and so on.
Atomic number
the atomic number of an atom is determined by the number of protons present
in the nucleus of that particular atom. it is denoted by Z. For hydrogen Z
= 1, for carbon Z = 6.
Mass number
Mass number of an atom can be determined by the sub atomic particles of an
atom that is the neutron and proton alone. The neutrons and protons make up
the nucleon therefore the mass of an atom resides in its nucleus.
The atomic number, mass number and symbol of an element can be
written as:
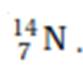
Isotopes
The atoms of some element have same atomic number but different mass
number, those are called the isotopes of that particular element. three
atomic species protium, deuterium, tritium has the same atomic number as 1
but mass number is 1, 2 and 3 respectively. These are the isotopes of
Hydrogen.
Application
An isotope of Uranium is used as a fuel in nuclear reactor.
An isotope of Cobalt is used in the treatment of cancer.
An isotope of iodine is used in the treatment of goitre.
Isobar
Two elements like calcium with atomic number 20 and Argon with atomic
number 18 have different number of electrons in these atoms but have the
same mass number 40. So, atoms of different elements with different atomic
number but the same mass number are known as isobars.