Pure Substance
When a substance has all the constituent particles same and shares a
similar chemical nature it is called as a pure substance. in other words,
we can tell a substance is a pure single form of matter. A pure substance
can be element or compound.
Mixture
When two or more kind of pure form of matter are mixed together it forms a
mixture. Such that when water and sodium chloride is mixed together then
sodium chloride can be separated from water by physical process, but sodium
and chloride is a pure substance and can be separated only by chemical
process.
Types of mixture
The types of mixture depend upon the nature of different components by
which the mixture is formed.
Homogeneous mixture
A mixture which has a uniform composition throughout such mixtures are
called as homogeneous mixture or a solution. salt dissolved in water is an
example of solution.
Heterogeneous mixture
A mixture which contains physically distinct part and have non-uniform
composition are called as heterogeneous mixture. oil and water is an
example of heterogeneous mixture.
Solution
A solution is a homogeneous mixture of two or more substance it has a
solvent and a solute as its component. Generally, a liquid either
containing a dissolved solid, liquid or gas is termed as a solution. But a
solution can also be a solid solution or maybe a gaseous solution. An alloy
is an example of solid solution and air is an example of gaseous solution.
salt mixed in water is an example of normal solution.
Alloy
Is a mixture of two or more metals or a metal and a nonmetal that cannot be
separated by any physical method. Brass is an example of alloy which
contains 30% zinc and 70% copper.
Properties of solution
Homogeneous mixture.
Particles of a solution are smaller than 1 NM in diameter.
Does not scatter a beam of light passing through them because of very small
particle size.
Solute cannot be separated from a solvent by the process of filtration.
Concentration of a solution
It is the amount of solute dissolved in a solution. relative proportion of
solute and solvent can be varied. Solution can be dilute concentrated or
saturated.
Various ways of expressing the concentration of a solution
Mass by mass percentage of a solution
=
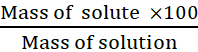
Mass by volume percentage of a solution
=
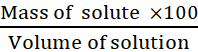
Volume by volume percentage of a
=
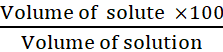
Dilute solution
When the amount of solute mixed in a solvent is low then it is called
dilute solution.
Concentrated solution
When the amount of solute mixed in a solvent is high it is called as a
concentrated solution.
Saturated solution.
At particular temperature the amount of dissolved solute in a solution is
to such an extent that no more solute can get dissolved at that particular
temperature in the solution than it is called a saturated solution.
Suspension
It is a non-Homogeneous system, in this system we notice that solid are
dispersed in liquid. In this the solid particles do not dissolve but remain
suspended throughout the bulk of the medium. suspended particles are
visible with naked eyes. Chalk powder mixed in water is an example of
suspension.
Properties of suspension
Heterogeneous in nature
Particles can be seen by naked eyes.
Scatter a Beam of light when passed through it
Solute particles settle down if left undisturbed and can be separated by
the process of filtration. It is unstable in nature.
Colloidal solution
In this type of solution the particles are and uniformly spread throughout
the solution. but due to the smaller size of the particles the mixture
appears to be homogeneous. Milk dissolved in water is an example of
colloidal solution.
Properties of colloid
Heterogeneous mixture.
Size of particle too small cannot be viewed by naked eyes.
Particle size Big enough to scatter beam of light passing through it.
Do not settle down when left undisturbed. it is stable in nature.
Separating components of a mixture
Different Method of separation can be used for the separation of individual
components from a mixture. Heterogeneous Mixture can be separated by simple
physical methods like hand picking, sieving, filtration, etc.
Separation by Evaporation
We can separate the volatile component(solution) from its non-volatile
solute by the method of evaporation. solution of Dye and water and the dye
can be separated from the water by using the process of evaporation.
Separation by centrifugation
In some mixture solid particles cannot be separated by the filtration
technique as the particles are very small. by the process of centrifugation
those can be separated. In this process the denser particles are send to
the bottom and the lightest particle stay at the top when they are spun
rapidly.
Application
Diagnostic Laboratories for blood and urine test.
Used in Diaries and home to separate butter from cream.
Used in washing machine to squeeze water out from the wet clothes.
Separation of two immiscible liquids
This process of separation can be done by using a separating funnel. the
principle that works is immiscible liquids separate out in layers depending
on their densities. oil can be separated from water by this process. In the
separating funnel once the water is dripped down slowly the stopper is
applied and the oil above remains back in the funnel.
Application
Separate mixture of oil and water
Extraction of iron from its ore
Separation of salt and camphor
To separate such mixtures that contain a sublimable volatile component from
a non sublimable impurity the sublimation process is used.
Here in this case we know that camphor changes directly from solid state to
the gaseous state so it can be removed from the salt by heating the mixture
in a funnel such that the camphor gets accumulated in the cooler part of
the inverted funnel.
Separation of different colours in a dye
The different colours used in a die can be separated by using the technique
of chromatography. Kroma in Greek means colour. In this
technique different types of solute dissolved in the same solvent can be
separated easily.
Application
Separate different colours in a dye.
Separate pigments from natural colour.
Separate drugs from blood.
Separation of a mixture of two miscible liquids
To separate two miscible liquids the method of distillation
is used. in this process the mixture is heated up to the boiling point such
that the boiling point of one liquid is different from the boiling point of
the other liquid and then the liquid with lower boiling point starts to
evaporate faster which is then captured by cooling down the evaporated
liquid in a separate container.
Separate different gases from air
Homogeneous mixture of gases can be separated into its components by fractional distillation. In this we have to take a volume
of air then compress and cool by increasing the pressure and decreasing
temperature until it turns into liquid air and then warm it up slowly so
that fractional distillation column can separate the different gases at
different temperature because all the different components of the mixture
shares have a different boiling point. Example: oxygen -183o C
and argon -186o C.
Obtain pure copper sulphate from an impure sample
Pure copper sulphate can be obtained from the impure sample by the process
of crystallization which is used to purify solids. In this
process pure solid are separated from its solution in the form of crystals.
It is a better process than evaporation because some solid may decompose on
heating or some impurities may remain dissolved in the solution.
Application
Purification of salt that we get from seawater.
Separation of Crystal of alum from impure samples
Physical and chemical change
Physical Change
Change of State is a physical change because these changes occur without
the change in composition that is no change in the chemical nature of the
substance. Ice, water and water vapour display different physical property
but have the same chemical property.
Chemical Change
Chemical changes bring change in chemical property of matter and that gives
us a new substance. Chemical change is also called as a chemical reaction
because in this process one substance react with another to undergo a
change in chemical composition.
Different type of pure substance
Element
Elements can be normally divided into metals, nonmetals and metalloids.
Metals have the following properties
Metals have lustre or shine
They have silvery grey or Golden yellow colour
Good conductor of heat and electricity
They are ductile
They are malleable
They are sonorous
Nonmetals have the following property
Display a variety of colour
Poor conductor of heat and electricity
Not lustrous, sonorous and malleable.
Compounds
Compound is a substance containing two or more elements chemically combined
with one another in a fixed proportion. The composition, colour and texture
of a compound is same throughout. the properties of a compound is totally
different compared to the properties of the combining elements.
|
MIXTURE
|
COMPOUND
|
|
Elements or compounds just mix together to form a mixture
and no new compound is formed.
|
Elements react to form new compounds.
|
|
A mixture has a variable composition
|
The composition of each new substance is always fixed.
|
|
A mixture shows the properties of the properties.
|
The new substance has totally different constituent
substances.
|
|
The constituents can be separated fairly easily by physical
methods
|
The constituents can be separated only by chemical or
electrochemical reaction
|